London Dispersion Forces Are Caused By
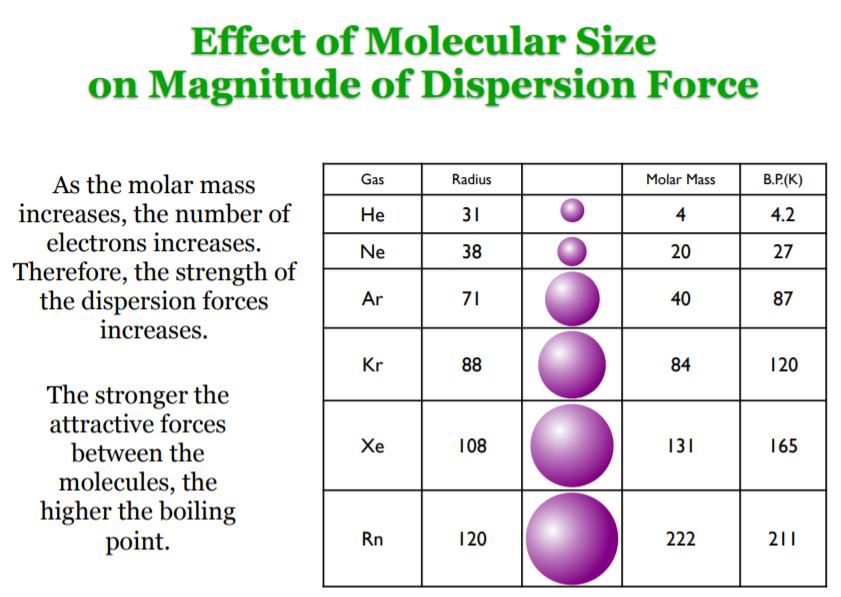
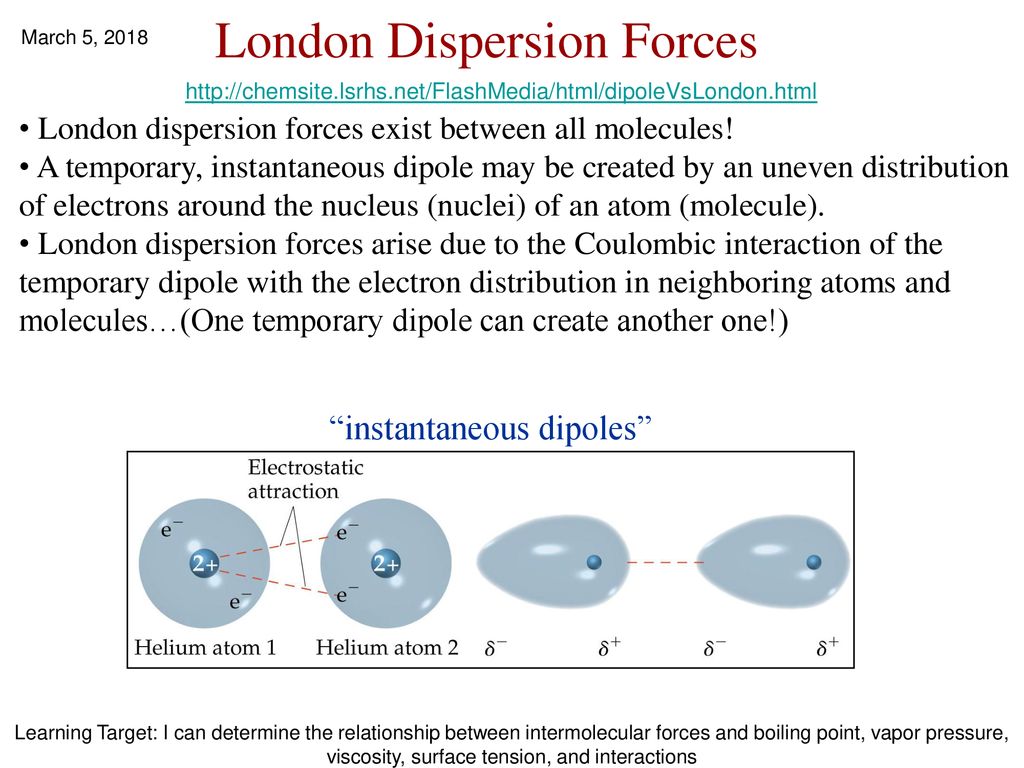
London forces are the attractive forces that cause non polar substances to condense to liquids and to freeze into solids when the temperature is lowered sufficiently.
London dispersion forces are caused by. Even though it is weak of the three van der waals forces orientation induction and dispersion the dispersion forces are usually dominant. The electrons that orbit molecules can move and have different charge distributions over time. These interactions are generally called dispersion forces. London forces are the attractive forces that cause nonpolar substances to condense to liquids and to freeze into solids when the temperature is lowered sufficiently.
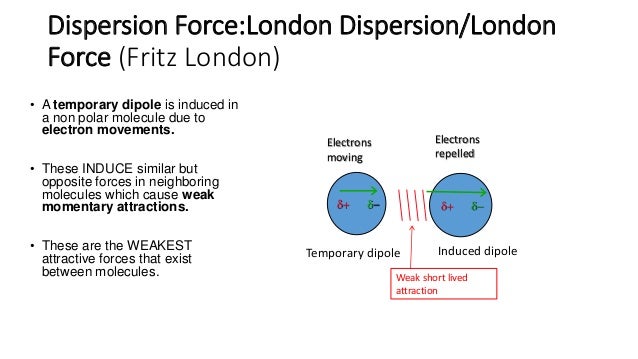
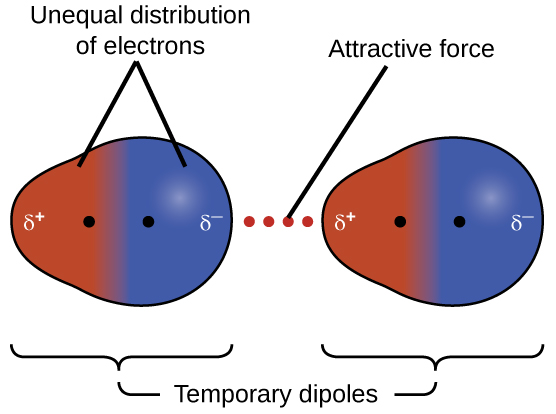
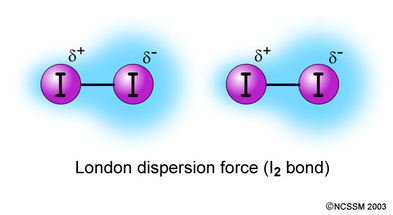
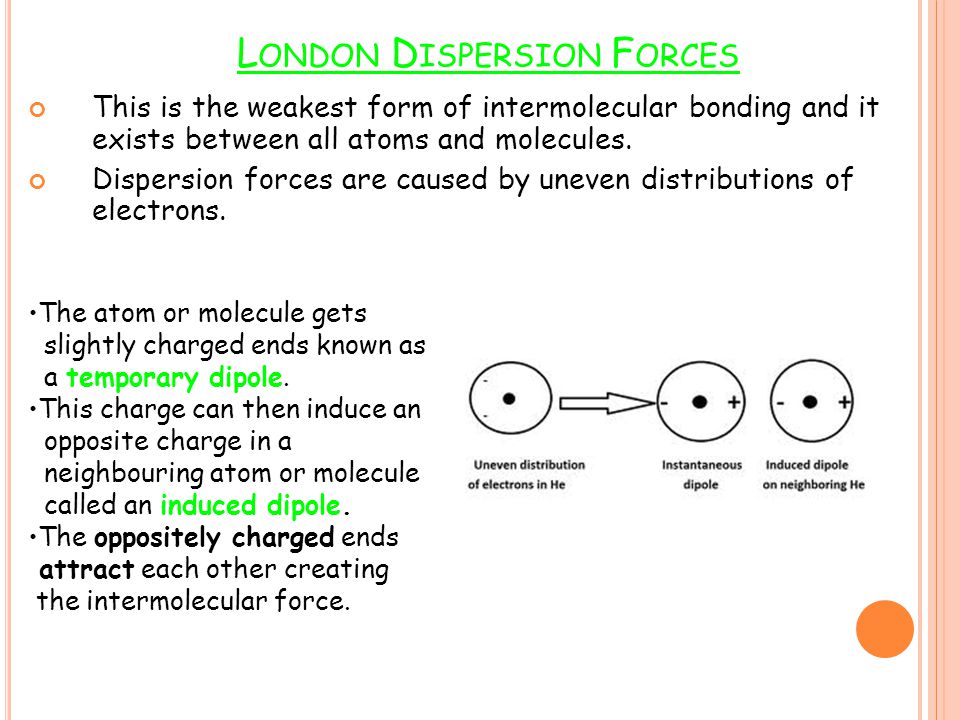
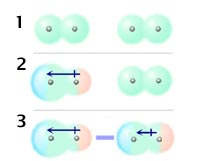
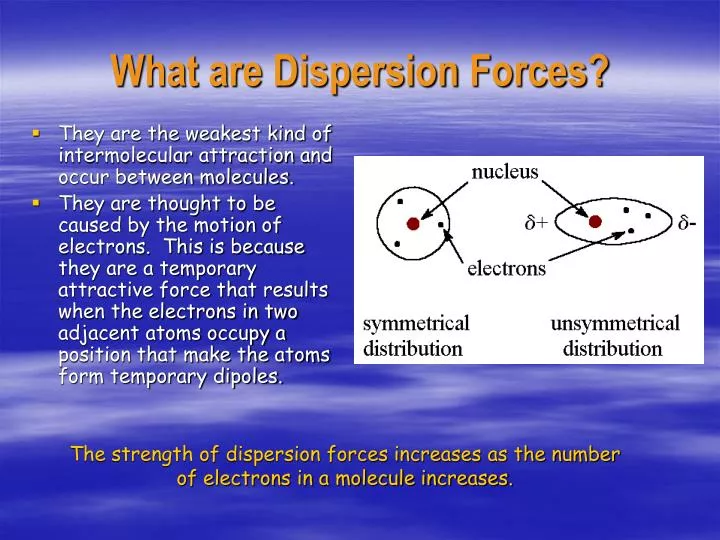
Because of the constant motion of the electrons an atom or molecule can develop a temporary instantaneous dipole when its electrons are distributed unsymmetrically about the nucleus. The london dispersion force is the weakest intermolecular force. The london dispersion force is the weakest intermolecular force. They are part of the van der waals forces.
Neighbouring atoms and molecules called dispersion forces 2 the induction effect by which polar molecules those having an asymmetrical distribution of electrons bring about a charge asymmetry in other molecules 3 an orientation effect caused by the mutual attraction of polar molecules resulting from alignment of dipoles. The ldf is named after the german american physicist fritz london. Because of the constant motion of the electrons an atom or molecule can develop a temporary instantaneous dipole when its electrons are distributed unsymmetrically about. A temporary dipole exists when you have two opposite charges.
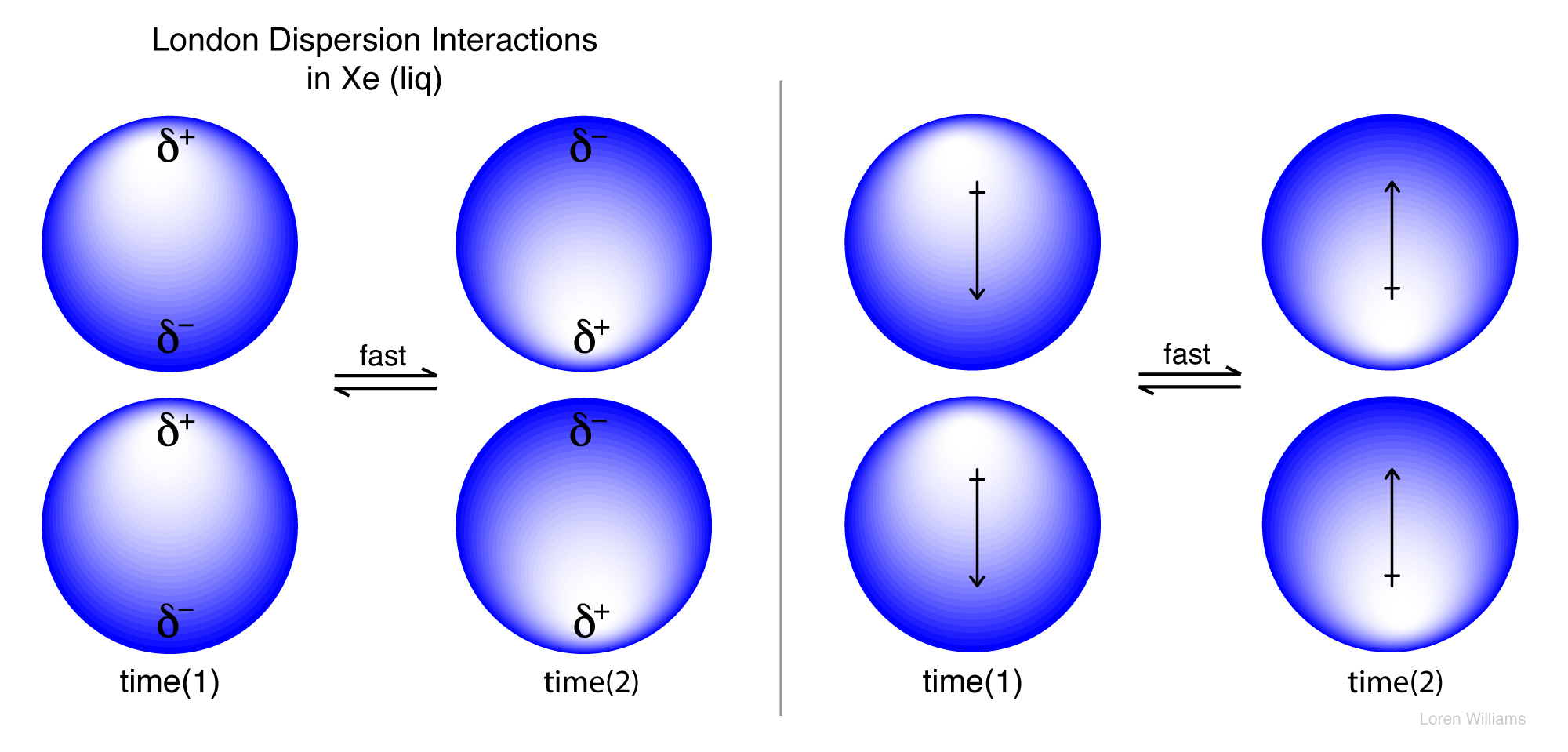
Instantaneous dipole induced dipole forces or london dispersion forces. Everyday chemistry london dispersion forces chemistry stack exchange london dispersion forces are responsible for the fact that non polar substances can be condensed to form liquids and sometimes solids at low temperatures i learned that what results in london. The dispersion forces are weak while the dipole dipole forces are stronger. One end of the molecule can be positive while the other end can be negative.
It is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles.