London Dispersion Forces Solubility In Water

Dispersion forces london forces weak intermolecular forces van der waal s forces.
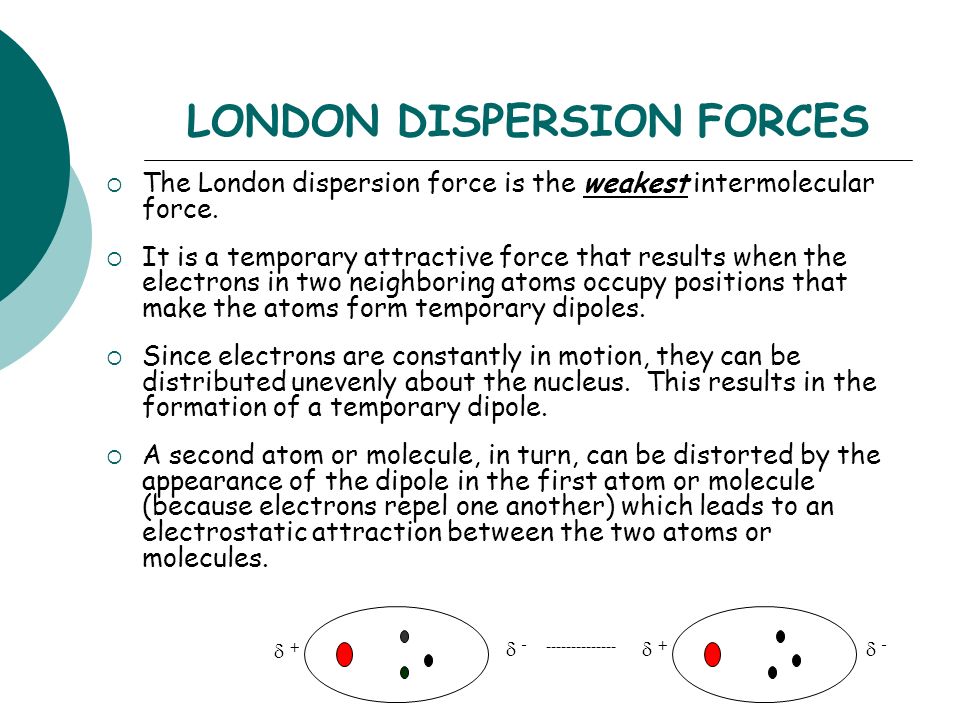
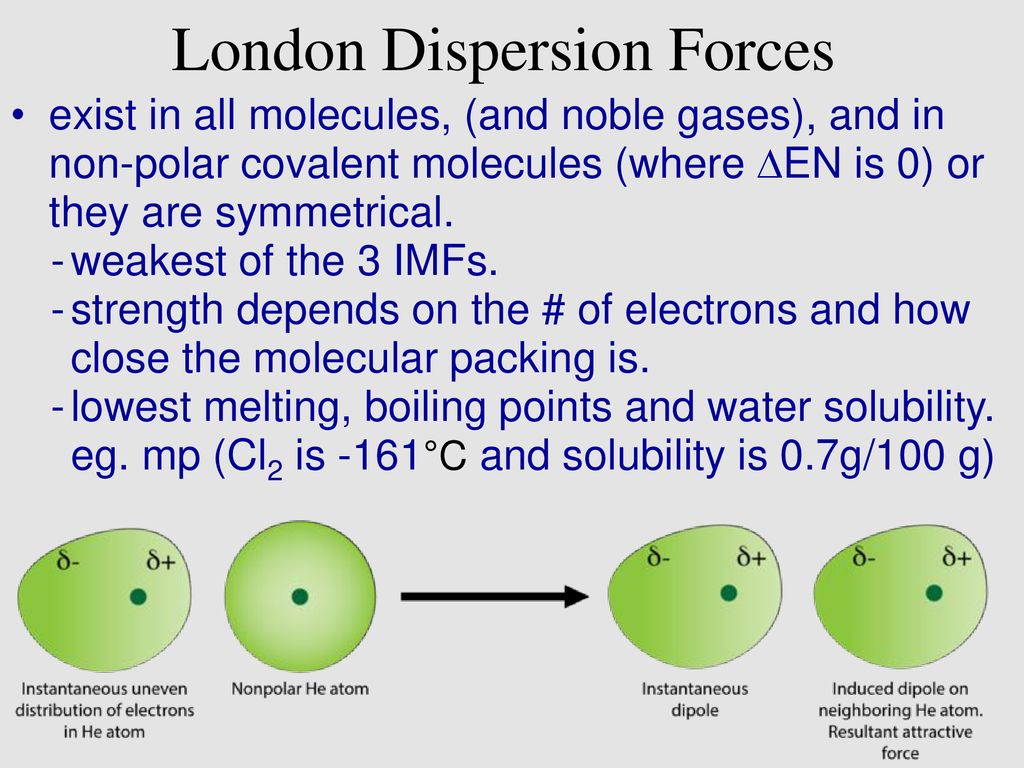
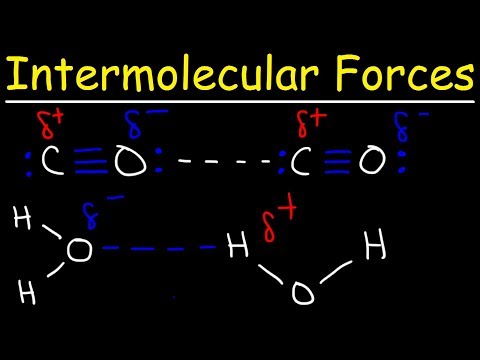
London dispersion forces solubility in water. Most but not all ionic compounds are quite soluble in water. Polar water molecules can interact only very weakly with non polar octane molecules that is the intermolecular force of attraction between an octane molecule and a water molecule is the very weak dispersion force. London dispersion forces ldf also known as dispersion forces london forces instantaneous dipole induced dipole forces or loosely van der waals forces are a type of force acting between atoms and molecules. The london dispersion force is the weakest intermolecular force.
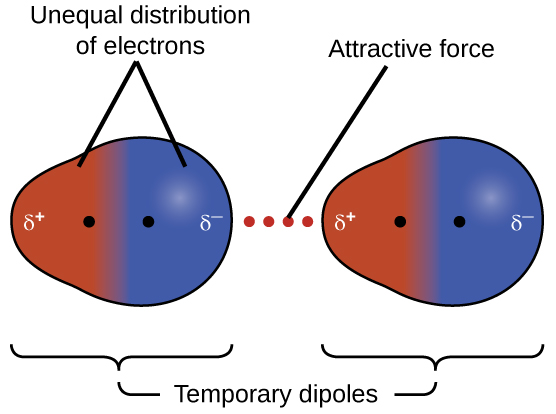
So in general if you think about solubility of a solute in water or especially if you think of a solid solute which is sodium chloride into a liquid solvent then the higher the temperature while you re in the liquid state the more of the solid you re going to be able to get into the liquid or you re going to raise solubility. The ldf is named after the german american physicist fritz london. The london dispersion force is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles. Table below summarizes some of the differences between ionic and molecular compounds.
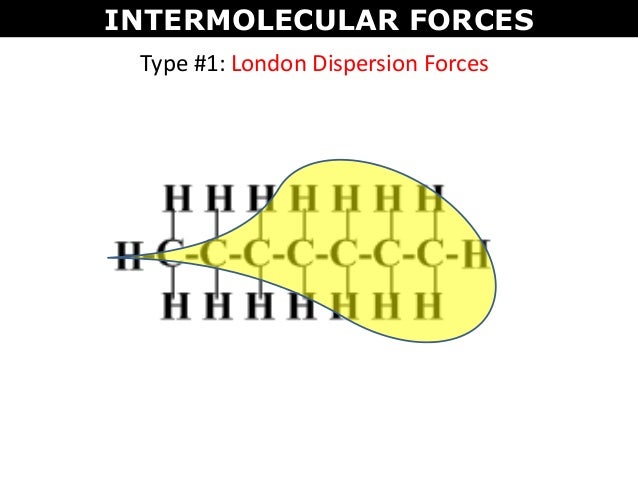
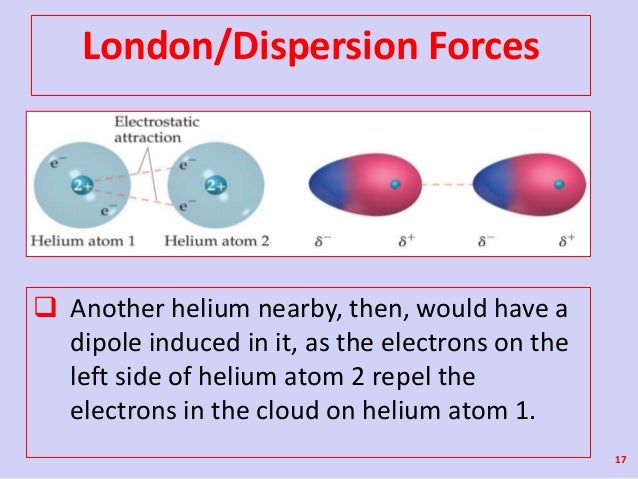
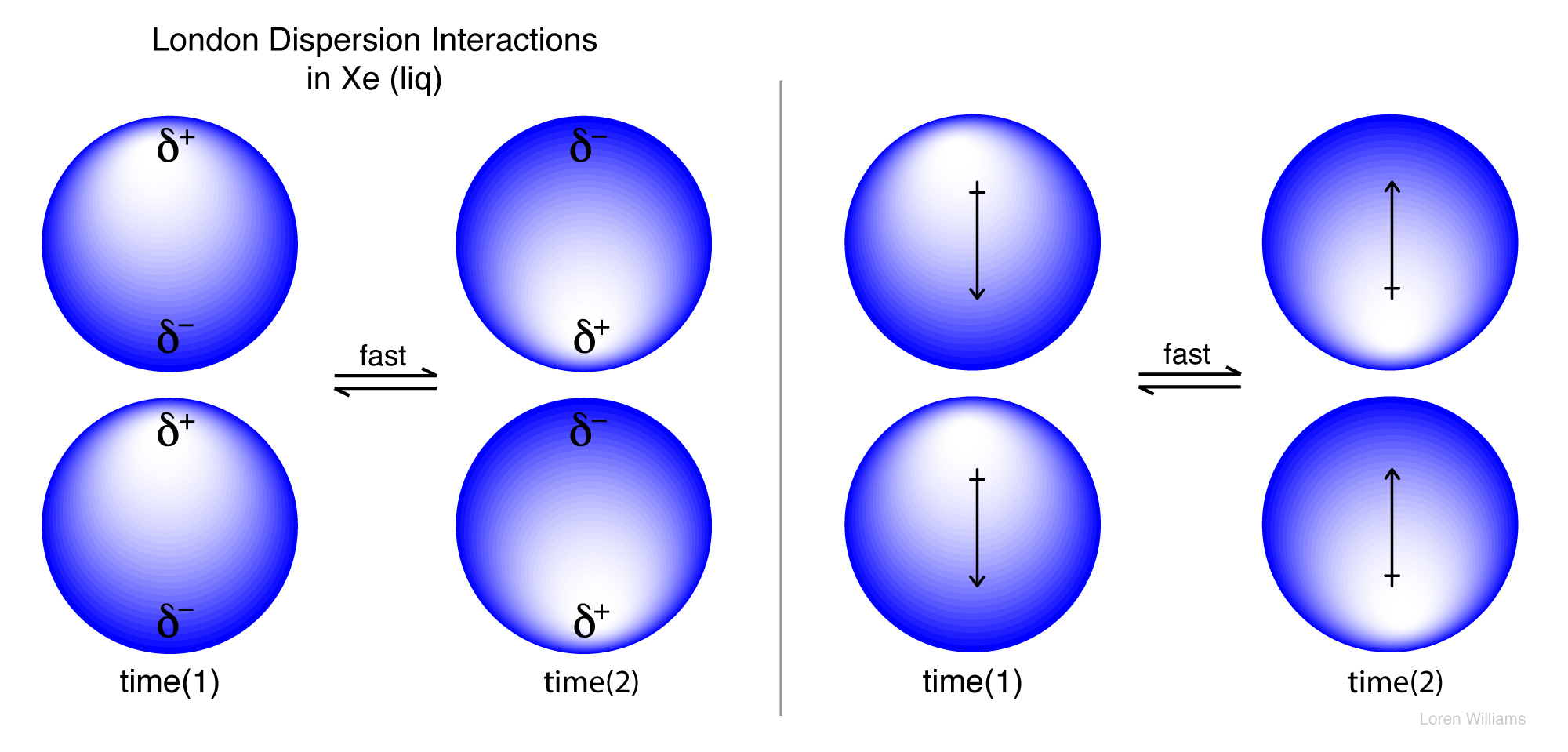
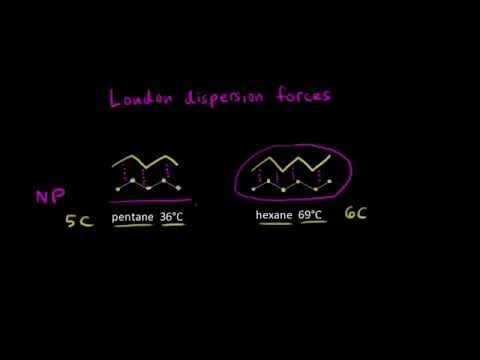
As the strength of forces decreases so do the melting points boiling points and solubility in water. If the molecule has more mass it means that the number of electrons present is greater and thus gives more strength to the dispersion forces that require more energy to break them and these molecules will have higher melting and boiling points than others. They are part of the van der waals forces. This force is sometimes called an induced dipole induced dipole attraction.
Substances that exhibit hydrogen bonding or dipole dipole forces are generally water soluble whereas those that exhibit only london dispersion forces are generally insoluble. Dispersion forces are very weak forces of attraction that act between all molecules and result from. Besides that the vapor pressure and the solubility in nonpolar solvents also increases.